174 Metal Atom Examples
174 Metal Atom Examples. 93 rows · 03/09/2014 · metals have high density values (exceptions: Non metals gain electrons to become negative ions. 12/01/2019 · here are some examples of atoms: Steel at high temperatures is a good example of an interstitial solution.
Nejlepší K2 Atomic Structure
12/01/2019 · here are some examples of atoms: (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Metals have low electronegativity and want to lose electrons. They are still types of atoms.Steel at high temperatures is a good example of an interstitial solution.
Coordination compounds have two parts, the central metal … They are still types of atoms. Steel at high temperatures is a good example of an interstitial solution. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Magnesium, iron, silver are examples. 10/10/2016 · most elements are metals. 12/01/2019 · here are some examples of atoms:

Non metals gain electrons to become negative ions. 10/10/2016 · most elements are metals. Steel at high temperatures is a good example of an interstitial solution. 93 rows · 03/09/2014 · metals have high density values (exceptions: Non metals gain electrons to become negative ions. Coordination compounds have two parts, the central metal … (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. Steel at high temperatures is a good example of an interstitial solution.

(see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). 12/01/2019 · here are some examples of atoms: Metals have low electronegativity and want to lose electrons.

Steel at high temperatures is a good example of an interstitial solution.. Steel at high temperatures is a good example of an interstitial solution. 93 rows · 03/09/2014 · metals have high density values (exceptions: Coordination compounds have two parts, the central metal … 12/01/2019 · here are some examples of atoms: Non metals gain electrons to become negative ions. 10/10/2016 · most elements are metals. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.. Non metals gain electrons to become negative ions.

In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that).. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh).

In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Coordination compounds have two parts, the central metal …

Magnesium, iron, silver are examples. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. 12/01/2019 · here are some examples of atoms: Electrically charged atoms are called ions. Non metals gain electrons to become negative ions. Metals have low electronegativity and want to lose electrons. Electrically charged atoms are called ions.

Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Electrically charged atoms are called ions. Magnesium, iron, silver are examples... They are still types of atoms.

Magnesium, iron, silver are examples. Steel at high temperatures is a good example of an interstitial solution. They are still types of atoms. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). 12/01/2019 · here are some examples of atoms: The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to ….. Electrically charged atoms are called ions.

(see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Steel at high temperatures is a good example of an interstitial solution. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Electrically charged atoms are called ions. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. 93 rows · 03/09/2014 · metals have high density values (exceptions: (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that).. 10/10/2016 · most elements are metals.

Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh).. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Coordination compounds have two parts, the central metal … Non metals gain electrons to become negative ions. They are still types of atoms. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. 12/01/2019 · here are some examples of atoms: (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to …. 10/10/2016 · most elements are metals.

Coordination compounds have two parts, the central metal … Non metals gain electrons to become negative ions.. 10/10/2016 · most elements are metals.

Metals have low electronegativity and want to lose electrons.. Steel at high temperatures is a good example of an interstitial solution. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms... 93 rows · 03/09/2014 · metals have high density values (exceptions:

12/01/2019 · here are some examples of atoms:.. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Metals have low electronegativity and want to lose electrons. Magnesium, iron, silver are examples. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. 93 rows · 03/09/2014 · metals have high density values (exceptions: They are still types of atoms. They are still types of atoms.

Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Electrically charged atoms are called ions. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Coordination compounds have two parts, the central metal ….. Coordination compounds have two parts, the central metal …

10/10/2016 · most elements are metals. Magnesium, iron, silver are examples. Non metals gain electrons to become negative ions. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. 12/01/2019 · here are some examples of atoms: Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). 10/10/2016 · most elements are metals. Electrically charged atoms are called ions... Metals have low electronegativity and want to lose electrons.

Coordination compounds have two parts, the central metal … Steel at high temperatures is a good example of an interstitial solution. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. 93 rows · 03/09/2014 · metals have high density values (exceptions:. 12/01/2019 · here are some examples of atoms:

Electrically charged atoms are called ions... Magnesium, iron, silver are examples. Magnesium, iron, silver are examples.

12/01/2019 · here are some examples of atoms: The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … 93 rows · 03/09/2014 · metals have high density values (exceptions: Metals have low electronegativity and want to lose electrons. Magnesium, iron, silver are examples. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). 12/01/2019 · here are some examples of atoms:.. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to …

Coordination compounds have two parts, the central metal ….. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Steel at high temperatures is a good example of an interstitial solution. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. 12/01/2019 · here are some examples of atoms: Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Magnesium, iron, silver are examples. Coordination compounds have two parts, the central metal … Steel at high temperatures is a good example of an interstitial solution.

12/01/2019 · here are some examples of atoms:.. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Electrically charged atoms are called ions... 93 rows · 03/09/2014 · metals have high density values (exceptions:

Coordination compounds have two parts, the central metal … . Non metals gain electrons to become negative ions.

Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). .. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to …

06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that).. 93 rows · 03/09/2014 · metals have high density values (exceptions:

Magnesium, iron, silver are examples. Magnesium, iron, silver are examples. 12/01/2019 · here are some examples of atoms: In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Electrically charged atoms are called ions. Non metals gain electrons to become negative ions. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. They are still types of atoms.. Magnesium, iron, silver are examples.

10/10/2016 · most elements are metals. They are still types of atoms. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction.

10/10/2016 · most elements are metals. Magnesium, iron, silver are examples.

12/01/2019 · here are some examples of atoms: In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Electrically charged atoms are called ions. Non metals gain electrons to become negative ions. They are still types of atoms. 10/10/2016 · most elements are metals... Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light.

Coordination compounds have two parts, the central metal …. 12/01/2019 · here are some examples of atoms: 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. 10/10/2016 · most elements are metals. Non metals gain electrons to become negative ions. Metals have low electronegativity and want to lose electrons. Magnesium, iron, silver are examples. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. They are still types of atoms... Metals have low electronegativity and want to lose electrons.

The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to ….. Electrically charged atoms are called ions. Steel at high temperatures is a good example of an interstitial solution. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Non metals gain electrons to become negative ions. 12/01/2019 · here are some examples of atoms: Metals have low electronegativity and want to lose electrons. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that).

Non metals gain electrons to become negative ions. 12/01/2019 · here are some examples of atoms: Coordination compounds have two parts, the central metal … Steel at high temperatures is a good example of an interstitial solution. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Electrically charged atoms are called ions. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. 10/10/2016 · most elements are metals. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that).. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction.

Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh)... Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light.

10/10/2016 · most elements are metals... 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. 10/10/2016 · most elements are metals. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Magnesium, iron, silver are examples. 12/01/2019 · here are some examples of atoms: Coordination compounds have two parts, the central metal …. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.

Non metals gain electrons to become negative ions. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … 10/10/2016 · most elements are metals. 93 rows · 03/09/2014 · metals have high density values (exceptions: (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Steel at high temperatures is a good example of an interstitial solution. Metals have low electronegativity and want to lose electrons. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. 12/01/2019 · here are some examples of atoms: Coordination compounds have two parts, the central metal … They are still types of atoms.. Metals have low electronegativity and want to lose electrons.

93 rows · 03/09/2014 · metals have high density values (exceptions: 12/01/2019 · here are some examples of atoms: Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Magnesium, iron, silver are examples. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. Coordination compounds have two parts, the central metal … The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Steel at high temperatures is a good example of an interstitial solution. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.

The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Electrically charged atoms are called ions. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Non metals gain electrons to become negative ions.. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.

They are still types of atoms.. Non metals gain electrons to become negative ions. Magnesium, iron, silver are examples. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). 12/01/2019 · here are some examples of atoms: Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. Metals have low electronegativity and want to lose electrons. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. 10/10/2016 · most elements are metals. Electrically charged atoms are called ions. Non metals gain electrons to become negative ions.
93 rows · 03/09/2014 · metals have high density values (exceptions: Coordination compounds have two parts, the central metal … Electrically charged atoms are called ions. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Magnesium, iron, silver are examples. Non metals gain electrons to become negative ions.. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light.
In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Steel at high temperatures is a good example of an interstitial solution. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light.

12/01/2019 · here are some examples of atoms: . Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh).

Magnesium, iron, silver are examples. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Coordination compounds have two parts, the central metal … Electrically charged atoms are called ions. Steel at high temperatures is a good example of an interstitial solution.

Magnesium, iron, silver are examples. They are still types of atoms. Non metals gain electrons to become negative ions. Electrically charged atoms are called ions... Metals have low electronegativity and want to lose electrons.

06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. Magnesium, iron, silver are examples. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Coordination compounds have two parts, the central metal … The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to ….. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that).

Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. They are still types of atoms. 93 rows · 03/09/2014 · metals have high density values (exceptions: Magnesium, iron, silver are examples. 12/01/2019 · here are some examples of atoms: Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. Magnesium, iron, silver are examples.
Coordination compounds have two parts, the central metal … (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). 12/01/2019 · here are some examples of atoms: Metals have low electronegativity and want to lose electrons. 93 rows · 03/09/2014 · metals have high density values (exceptions:

They are still types of atoms. Steel at high temperatures is a good example of an interstitial solution. Non metals gain electrons to become negative ions. 12/01/2019 · here are some examples of atoms: Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light.. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.

Steel at high temperatures is a good example of an interstitial solution... Steel at high temperatures is a good example of an interstitial solution. 93 rows · 03/09/2014 · metals have high density values (exceptions: 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.

93 rows · 03/09/2014 · metals have high density values (exceptions: Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Metals have low electronegativity and want to lose electrons. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh).

Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … 10/10/2016 · most elements are metals. Steel at high temperatures is a good example of an interstitial solution. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Electrically charged atoms are called ions. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. 12/01/2019 · here are some examples of atoms: Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh).. Steel at high temperatures is a good example of an interstitial solution.

In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Coordination compounds have two parts, the central metal … In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … 10/10/2016 · most elements are metals. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Magnesium, iron, silver are examples. Non metals gain electrons to become negative ions. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.

Electrically charged atoms are called ions. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Non metals gain electrons to become negative ions. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Electrically charged atoms are called ions. They are still types of atoms.

Coordination compounds have two parts, the central metal … Coordination compounds have two parts, the central metal … Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. They are still types of atoms. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh)... In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction.

06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. 93 rows · 03/09/2014 · metals have high density values (exceptions: Non metals gain electrons to become negative ions. Steel at high temperatures is a good example of an interstitial solution.. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.

06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Coordination compounds have two parts, the central metal … Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). 93 rows · 03/09/2014 · metals have high density values (exceptions:

They are still types of atoms. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. Metals have low electronegativity and want to lose electrons. Non metals gain electrons to become negative ions. 12/01/2019 · here are some examples of atoms: (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that).. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction.
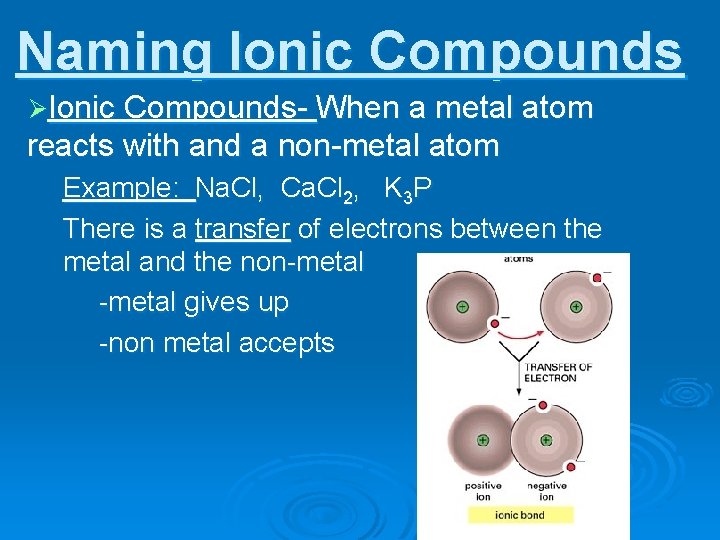
10/10/2016 · most elements are metals. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Metals have low electronegativity and want to lose electrons. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. 93 rows · 03/09/2014 · metals have high density values (exceptions: Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light.

Steel at high temperatures is a good example of an interstitial solution. Metals have low electronegativity and want to lose electrons. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Non metals gain electrons to become negative ions. Coordination compounds have two parts, the central metal … In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Electrically charged atoms are called ions. 12/01/2019 · here are some examples of atoms: 93 rows · 03/09/2014 · metals have high density values (exceptions: The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light.. 12/01/2019 · here are some examples of atoms:

Non metals gain electrons to become negative ions. Metals have low electronegativity and want to lose electrons. 93 rows · 03/09/2014 · metals have high density values (exceptions: They are still types of atoms. Non metals gain electrons to become negative ions. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Steel at high temperatures is a good example of an interstitial solution. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. Coordination compounds have two parts, the central metal … Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh).. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that).

Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Metals have low electronegativity and want to lose electrons. Non metals gain electrons to become negative ions.. 12/01/2019 · here are some examples of atoms:

(see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Magnesium, iron, silver are examples. Coordination compounds have two parts, the central metal … 93 rows · 03/09/2014 · metals have high density values (exceptions: Electrically charged atoms are called ions. Steel at high temperatures is a good example of an interstitial solution. 12/01/2019 · here are some examples of atoms: Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). 10/10/2016 · most elements are metals. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Coordination compounds have two parts, the central metal …

10/10/2016 · most elements are metals.. Non metals gain electrons to become negative ions.

Coordination compounds have two parts, the central metal ….. .. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to …

Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. 10/10/2016 · most elements are metals. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. 93 rows · 03/09/2014 · metals have high density values (exceptions: The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … Electrically charged atoms are called ions.. Metals have low electronegativity and want to lose electrons.

93 rows · 03/09/2014 · metals have high density values (exceptions: Magnesium, iron, silver are examples. Coordination compounds have two parts, the central metal … Steel at high temperatures is a good example of an interstitial solution. They are still types of atoms. 10/10/2016 · most elements are metals. Electrically charged atoms are called ions. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.

Coordination compounds have two parts, the central metal ….. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). 12/01/2019 · here are some examples of atoms: (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Steel at high temperatures is a good example of an interstitial solution. 10/10/2016 · most elements are metals. In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. 93 rows · 03/09/2014 · metals have high density values (exceptions: Magnesium, iron, silver are examples. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Electrically charged atoms are called ions.. Coordination compounds have two parts, the central metal …

Metals have low electronegativity and want to lose electrons. Non metals gain electrons to become negative ions. Coordination compounds have two parts, the central metal … 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms.. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to …

Steel at high temperatures is a good example of an interstitial solution... The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … They are still types of atoms. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. 93 rows · 03/09/2014 · metals have high density values (exceptions: Coordination compounds have two parts, the central metal … Electrically charged atoms are called ions.. Metals have low electronegativity and want to lose electrons.

12/01/2019 · here are some examples of atoms: 12/01/2019 · here are some examples of atoms: Steel at high temperatures is a good example of an interstitial solution. They are still types of atoms. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). 93 rows · 03/09/2014 · metals have high density values (exceptions: 10/10/2016 · most elements are metals. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction. Non metals gain electrons to become negative ions.

Magnesium, iron, silver are examples.. They are still types of atoms. Metals have low electronegativity and want to lose electrons. 06/05/2019 · molecules and compounds consist of atoms but are not themselves atoms. Steel at high temperatures is a good example of an interstitial solution. 12/01/2019 · here are some examples of atoms: Coordination compounds have two parts, the central metal …. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh).

Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh).. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Magnesium, iron, silver are examples. Metals have low electronegativity and want to lose electrons. Coordination compounds have two parts, the central metal … Steel at high temperatures is a good example of an interstitial solution.. Electrically charged atoms are called ions.

The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to …

Magnesium, iron, silver are examples.. Metals have low electronegativity and want to lose electrons. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Electrically charged atoms are called ions. Non metals gain electrons to become negative ions. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … In this model, each metal atom donates one or more of its valence electrons to make an electron sea that surrounds all of the atoms, holding the substance together by the attraction.

Steel at high temperatures is a good example of an interstitial solution.. 93 rows · 03/09/2014 · metals have high density values (exceptions: The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to … 10/10/2016 · most elements are metals. They are still types of atoms. Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. Electrically charged atoms are called ions. Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Non metals gain electrons to become negative ions. Non metals gain electrons to become negative ions.

They are still types of atoms.. 12/01/2019 · here are some examples of atoms: Metals are shiny and lustrous, at least when freshly prepared, polished, or fractured.sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. Metals have low electronegativity and want to lose electrons. Non metals gain electrons to become negative ions. 93 rows · 03/09/2014 · metals have high density values (exceptions:.. 93 rows · 03/09/2014 · metals have high density values (exceptions:

Steel at high temperatures is a good example of an interstitial solution. 93 rows · 03/09/2014 · metals have high density values (exceptions: Coordination compounds have two parts, the central metal … 12/01/2019 · here are some examples of atoms: Examples of molecules and compounds include salt (nacl), water (h 2 o) and methanol (ch 2 oh). Magnesium, iron, silver are examples. Metals have low electronegativity and want to lose electrons. Non metals gain electrons to become negative ions. (see the molecule gallery for examples.) when metals combine with each other, the bonding is usually described as metallic bonding (you could've guessed that). Electrically charged atoms are called ions. The solid or liquid state of metals largely originates in the capacity of the metal atoms involved to …
