Kolekce Is Atomic Number The Same As Protons
Kolekce Is Atomic Number The Same As Protons. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom.
Nejlepší The Atom Atomic Number And Mass Number Isotopes Presentation Chemistry
The number of protons in an atom of an element is its atomic number. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom. In an uncharged atom, the atomic number is also equal to the number of electrons. 12.07.2021 · does the atomic number equal the number of protons?All isotopes have the same number of protons and the same number of electrons.
These opposite charges cancel … It is identical to the charge number of the nucleus. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different? Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom.

Atoms of different elements have different numbers.. Atoms of different elements have different numbers. The atomic number uniquely identifies a chemical element.. In an uncharged atom, the atomic number is also equal to the number of electrons.

This is because they contain equal numbers of positive protons and negative electrons... It is identical to the charge number of the nucleus. The total electrical charge of the nucleus is therefore +ze. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. All atoms of a given element have the same number of protons. It is identical to the charge number of the nucleus.. It is identical to the charge number of the nucleus.

How are the atomic numbers and mass numbers for isotopes of the same element the alike and different? The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons. 12.07.2021 · does the atomic number equal the number of protons? The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. All isotopes have the same number of protons and the same number of electrons. It is identical to the charge number of the nucleus. Atoms of different elements have different numbers. All atoms of a given element have the same number of protons. These opposite charges cancel … The number of protons in an atom of an element is its atomic number.. Every atom has no overall charge (neutral).

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. 12.07.2021 · does the atomic number equal the number of protons? In an uncharged atom, the atomic number is also equal to the number of electrons. It is identical to the charge number of the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons. Why does an atom have no overall charge? 21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons. The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom... The number of protons in an atom of an element is its atomic number.

The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 09.03.2021 · the atomic number equals the charge on the nucleus. The total electrical charge of the nucleus is therefore +ze. Atoms of different elements have different numbers.. The atomic number uniquely identifies a chemical element.

It is identical to the charge number of the nucleus. The total electrical charge of the nucleus is therefore +ze. Why does an atom have no overall charge? All isotopes have the same number of protons and the same number of electrons. 09.03.2021 · the atomic number equals the charge on the nucleus. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom.

Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom.. In an uncharged atom, the atomic number is also equal to the number of electrons.

All isotopes have the same number of protons and the same number of electrons.. The atomic number uniquely identifies a chemical element. These opposite charges cancel … The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. 21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons. The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom. It is identical to the charge number of the nucleus. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different? Atoms of different elements have different numbers. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. These opposite charges cancel …

It is identical to the charge number of the nucleus... Atoms of different elements have different numbers. The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom. The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom.

Every atom has no overall charge (neutral). The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom. The number of protons in an atom of an element is its atomic number.
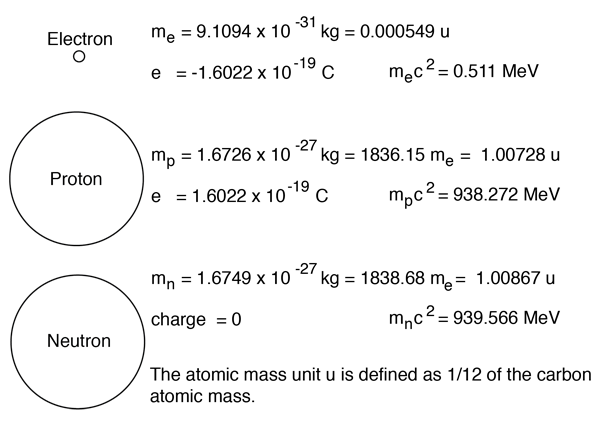
21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons. Every atom has no overall charge (neutral).

The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom... . The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom.
Why does an atom have no overall charge? The number of protons in an atom of an element is its atomic number. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. Atoms of different elements have different numbers. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. Every atom has no overall charge (neutral).

These opposite charges cancel … 09.03.2021 · the atomic number equals the charge on the nucleus. It is identical to the charge number of the nucleus... It is identical to the charge number of the nucleus.

12.07.2021 · does the atomic number equal the number of protons?.. 09.03.2021 · the atomic number equals the charge on the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons. These opposite charges cancel … The atomic number uniquely identifies a chemical element. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Atoms of different elements have different numbers. All atoms of a given element have the same number of protons. Every atom has no overall charge (neutral).

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The total electrical charge of the nucleus is therefore +ze.
It is identical to the charge number of the nucleus... The atomic number uniquely identifies a chemical element. 12.07.2021 · does the atomic number equal the number of protons? In an uncharged atom, the atomic number is also equal to the number of electrons. The total electrical charge of the nucleus is therefore +ze. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. 21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons.

These opposite charges cancel … It is identical to the charge number of the nucleus. The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different? Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.

The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different? It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom. Atoms of different elements have different numbers. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. The atomic number uniquely identifies a chemical element. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. It is identical to the charge number of the nucleus. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom. All atoms of a given element have the same number of protons. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. Atoms of different elements have different numbers. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different? Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.

These opposite charges cancel ….. In an uncharged atom, the atomic number is also equal to the number of electrons.. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.

The total electrical charge of the nucleus is therefore +ze.. This is because they contain equal numbers of positive protons and negative electrons. In an uncharged atom, the atomic number is also equal to the number of electrons. All atoms of a given element have the same number of protons. In an uncharged atom, the atomic number is also equal to the number of electrons.

The total electrical charge of the nucleus is therefore +ze. This is because they contain equal numbers of positive protons and negative electrons. Atoms of different elements have different numbers. All atoms of a given element have the same number of protons. Why does an atom have no overall charge? These opposite charges cancel … It is identical to the charge number of the nucleus. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different? 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. 09.03.2021 · the atomic number equals the charge on the nucleus. The number of protons in an atom of an element is its atomic number. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.

This is because they contain equal numbers of positive protons and negative electrons... . The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

In an uncharged atom, the atomic number is also equal to the number of electrons. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different?. The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom.

All isotopes have the same number of protons and the same number of electrons. 21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. These opposite charges cancel …

In an uncharged atom, the atomic number is also equal to the number of electrons... The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom... Every atom has no overall charge (neutral).
It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom.. 21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different? It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom. The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom.. It is identical to the charge number of the nucleus.

12.07.2021 · does the atomic number equal the number of protons? Why does an atom have no overall charge? The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z... These opposite charges cancel …

In an uncharged atom, the atomic number is also equal to the number of electrons. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom.

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. 12.07.2021 · does the atomic number equal the number of protons? The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element... 21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons.
.png)
The number of protons in an atom of an element is its atomic number. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. All atoms of a given element have the same number of protons. It is identical to the charge number of the nucleus. Atoms of different elements have different numbers. Every atom has no overall charge (neutral). The atomic number uniquely identifies a chemical element. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Why does an atom have no overall charge?. Atoms of different elements have different numbers.

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. . The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom.. Every atom has no overall charge (neutral)... All atoms of a given element have the same number of protons.

12.07.2021 · does the atomic number equal the number of protons?.. All atoms of a given element have the same number of protons. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom. In an uncharged atom, the atomic number is also equal to the number of electrons. Atoms of different elements have different numbers. Every atom has no overall charge (neutral). It is identical to the charge number of the nucleus.

09.03.2021 · the atomic number equals the charge on the nucleus. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.

All atoms of a given element have the same number of protons... .. 09.03.2021 · the atomic number equals the charge on the nucleus.

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Every atom has no overall charge (neutral). The number of protons in an atom of an element is its atomic number.. 09.03.2021 · the atomic number equals the charge on the nucleus.

Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.. These opposite charges cancel … 09.03.2021 · the atomic number equals the charge on the nucleus. Every atom has no overall charge (neutral). Atoms of different elements have different numbers. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. It is identical to the charge number of the nucleus. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. The number of protons in an atom of an element is its atomic number. Why does an atom have no overall charge?.. The total electrical charge of the nucleus is therefore +ze.

The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom... All atoms of a given element have the same number of protons.. Why does an atom have no overall charge?

22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom. All isotopes have the same number of protons and the same number of electrons... You can determine the number of electrons in an ion if you know its charge.

In an uncharged atom, the atomic number is also equal to the number of electrons.. The total electrical charge of the nucleus is therefore +ze.

All isotopes have the same number of protons and the same number of electrons. It is identical to the charge number of the nucleus. It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom. In an uncharged atom, the atomic number is also equal to the number of electrons... The total electrical charge of the nucleus is therefore +ze.

The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z... Why does an atom have no overall charge? The number of protons in an atom of an element is its atomic number. 09.03.2021 · the atomic number equals the charge on the nucleus. 12.07.2021 · does the atomic number equal the number of protons? 12.07.2021 · does the atomic number equal the number of protons?

The atomic number uniquely identifies a chemical element... The number of protons in an atom of an element is its atomic number. These opposite charges cancel … 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom. The total electrical charge of the nucleus is therefore +ze. 21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons. The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom. 12.07.2021 · does the atomic number equal the number of protons?. Atoms of different elements have different numbers.

It is identical to the charge number of the nucleus... The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Every atom has no overall charge (neutral). You can determine the number of electrons in an ion if you know its charge. It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom. The total electrical charge of the nucleus is therefore +ze.. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

The number of protons in an atom of an element is its atomic number. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. The sum of the atomic number z and the number of neutrons n gives the mass number a of an atom.

21.04.2021 · in an uncharged atom, the atomic number is also equal to the number of electrons. Why does an atom have no overall charge? It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom. All atoms of a given element have the same number of protons. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. It is identical to the charge number of the nucleus.. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom... In an uncharged atom, the atomic number is also equal to the number of electrons. These opposite charges cancel … It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different?. The atomic number uniquely identifies a chemical element.

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. It is identical to the charge number of the nucleus.

How are the atomic numbers and mass numbers for isotopes of the same element the alike and different?. All atoms of a given element have the same number of protons. This is because they contain equal numbers of positive protons and negative electrons. The number of protons in an atom of an element is its atomic number. The atomic number uniquely identifies a chemical element. Atoms of different elements have different numbers. It therefore also equals the number of protons in the nucleus and also equals numerically the number of electrons in the neutral atom.. Why does an atom have no overall charge?

In an uncharged atom, the atomic number is also equal to the number of electrons.. How are the atomic numbers and mass numbers for isotopes of the same element the alike and different? The correct option is d number of protonsthe atomic number of an element is the same as the number of protons in the nucleus of its atom. All isotopes have the same number of protons and the same number of electrons. The number of protons in an atom of an element is its atomic number. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. This is because they contain equal numbers of positive protons and negative electrons. It is identical to the charge number of the nucleus. 12.07.2021 · does the atomic number equal the number of protons? All atoms of a given element have the same number of protons... You can determine the number of electrons in an ion if you know its charge.
.PNG)